Identifying Cepaeas by the External Traits
Intro
This is a post about identifying the cepaea snails from photos and about all the little bits of knowledge that aren't explicitly recorded elsewhere. It will be long, very detailed, convoluted and it will mostly be of interest to those who already have some experience with Cepaea and ID them a lot. (I will tag some people who may be interested: feel free to add or disagree in the comments. I don't expect most of this info will be new to you, but maybe some of it will. @susanhewitt @sunnysnail @angus10 @mathijs_zonneveld @gural-sverlova)
So you have genus Cepaea and the two species, nemoralis and hortensis. How do you separate them? In principle you're supposed to look at the love arrows:

hortensis
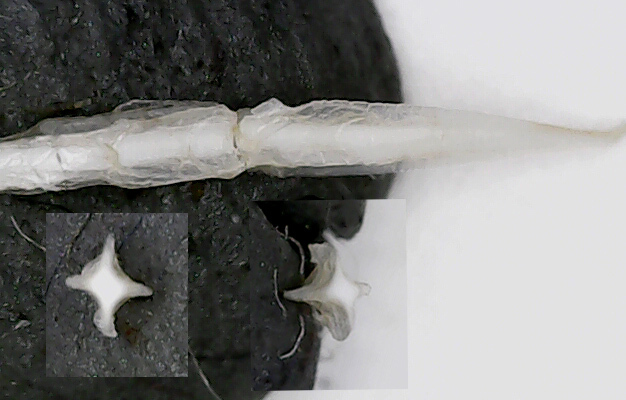
nemoralis
There are other anatomical differences as well. But on INaturalist it isn't going to happen. All you have are external photos. Even outside of context of INat there are times when all you find is an empty shell. Also, dissecting is icky. So can you reliably ID cepaeas at all? Yes, but there are complications. It's not so hopeless that you'd have to throw your hands in the air and say "no dissection = no ID", but also more complicated than just looking at the edge of the opening.
Primary Traits
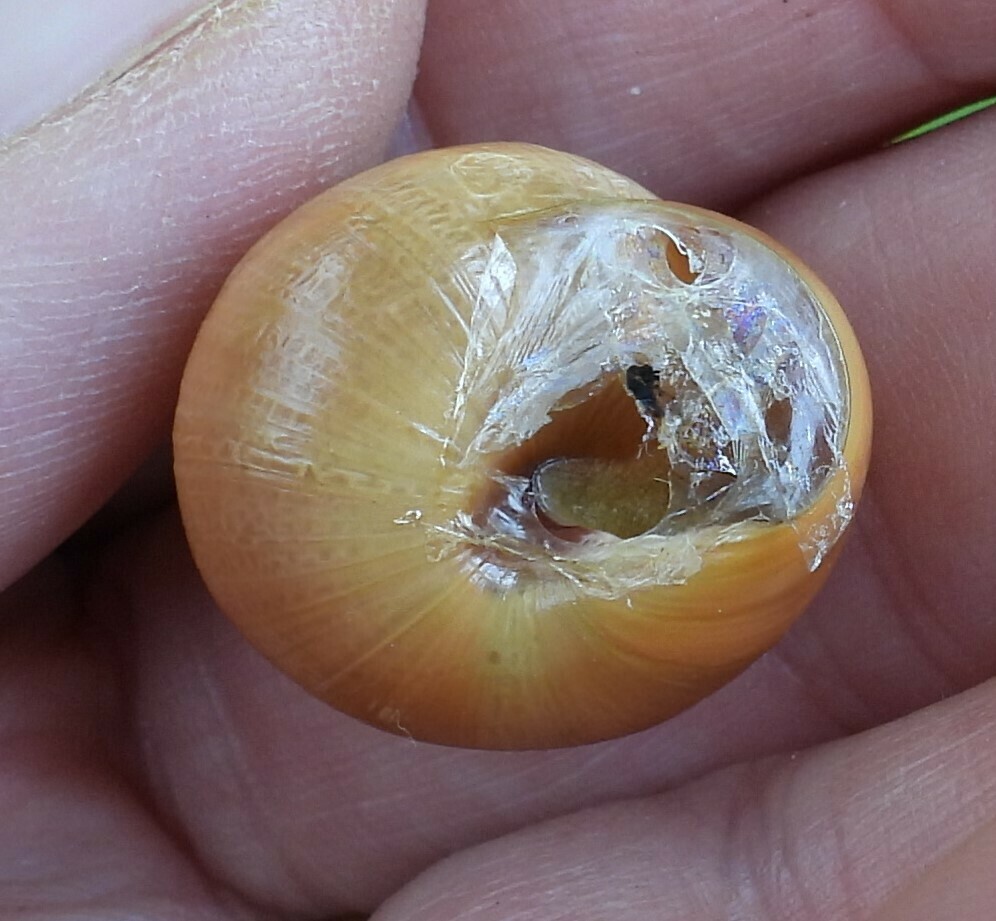
The first and the most basic thing you learn is that the two species differ by the color of the lip, that thing around the opening. Dark brown for nemoralis, white for hortensis, like this:

nemoralis

hortensis
This is good enough up to a point, but there are going to be problems with it.
The second thing you learn (and the first problem you encounter) is that the lip color is not totally reliable. There are "reverse color cepaeas" - white lipped nemoralis and black lipped hortensis.
For now I'm going to ignore this issue and treat "black/white lipped snail" as interchangeable with "nemoralis/hortensis", but I will return to this issue later in the Reverse Color Cepaeas section, where I will ask: how serious is this problem?
If you ignore the reverse color cepaeas, identifying starts to sounds simple again - you look at the color of the lip and base your ID on that, but unfortunately there are still further complications. If you're not aware of them you're going to badly misidentify lots and lots of specimens.
To start with: what is a lip, anyway? Is a lip simply the edge of the opening? No. A lip is a specific structure, a thickening of the shell that only mature snails develop. If the snail is not mature, there isn't going to be a lip. Then it doesn't matter what color you think you can see on the edge of the opening: it's irrelevant for identification purposes. How do you tell a difference?
This is largely covered in a good post by Susan J. Hewitt. I'm going to quote the most important bits:
"How can you tell if the snail is an adult? In snails of this genus (and in many other land-snail genera), once the snail reaches adulthood/sexual maturity, the shell stops growing any larger, and instead it grows thicker. In particular, the lip (the very edge of the opening of the shell) in adults becomes greatly strengthened, strongly reinforced, and also somewhat out-turned, a bit flared-out. So, in adults the lip of the shell is thick and strong, and it is out-turned to a certain degree."
"A live juvenile or subadult Cepaea snail that is active will always appear to have a white lip on the shell. But what you are seeing is usually the live mantle tissue which is wrapped over the edge of the shell, actively laying down more shell material. That is how the shell increases in size. And any brand-new shell material will also appear whitish, yellowish, or even transparent. This apparent pale lip is not an indication that the shell is mature."
This phenomenon gives rise to the "false hortensis" - immature cepaeas that superficially appear to have a white lip, leading to a very common identification mistake. For example, do you think these are hortensis?
If you were to think that, you'd be wrong - nemoralis all of them. The edge of the opening may appear white here, but it tells you nothing about the species. A lip hasn't developed here. What you a want to see is the opening 1) expanding outwards and 2) thickening, like this:
These are legitimate hortensis, or perhaps you may want to say "cepaeas with legitimate white lips".
What about these ones?
Here you can clearly see the opening expanding outwards. So are these mature hortensis? No, still not. Still not mature, although they are very close. The issue here is that the lip has already expanded but hasn't thickened yet. You can tell from the fact that it's partially translucent (you can see the faint "veins" through it). How else can you tell a difference? Compare a proper, totally mature specimen with a 99%-mature-but-not-quite-there one:

This is a real mature hortensis. You do not see the veins through the shell. Notice a pattern of color change on the lip: first a bright pale strip, then the very edge which seems a bit darker, whish is actually because it's faintly transparent. [observation][ImgLic]

This is an immature cepaea that may well be nemoralis. Notice the veins, notice the other pattern on the lip: first it's dark, (because it's transparent), then the very edge is light (it's also transparent, but here you can see the mantle through it), there's no clear white strip - this is a pattern of a not totally mature cepaea. Notice how this can also be seen in the 3 mature cepaeas vs 3 not totally mature ones above: totally mature ones have a defined white strip, in immature ones you have something darker and muddy looking. [observation][ImgLic]
If you think this represents too much caution, look at the little experiment Angus did:

Look at the snail with bands. The opening is expanded and the edge is white, has to be hortensis, right?

Then, 4 days later look at it again. Oh no.
When you have a very well-observed area with many thousands of cepaea photos where only nemoralis lives (eg. New York) out of many thousands one or two snails will be exactly, on the very edge of adulthood and look very, very convincingly like hortensis, but there will be only a couple of them. In cases like that, I think it's good to be extra strict and classify everything, except the completely and entirely convincing specimens and nemoralis. When there are many convincing hortensis observations, then the standards can perhaps be lowered a little. This, for the record, is why I think there are zero hortensis in New York city or Washington state.
Photos of "false hortensis" found their way into many places, see for example the Wikipedia media gallery - it's a mess: https://commons.wikimedia.org/wiki/Cepaea_hortensis. Many snails pictured there can't be confidently called hortensis, and some, in fact, may be pretty confidently called nemoralis. (In general Wikipedia image galleries are not well-identified. They don't have anything resembling a robust ID consensus system over there.)
At this point things may be starting to feel too gloomy: there are so many disclaimers about this whole lip thing, and it's so hard to tell - is it properly developed, is it not properly developed. Turned out some seemingly identifiable snails were unidentifiable, after all. Here are some good news though: in order to call a snail "nemoralis" what you want is not a black lip as such, but a presence of the black pigment. And fortunately for the identifiers, the black pigment begins to form on the columella of the shell before it forms on the lip, like this:
When you see something like that, it's safe to say you are holding nemoralis then. Knowing this can save many observations of juveniles from being unidentifiable. Of course, the absence of black pigment does not imply hortensis (except very weekly). On a young juvenile there will be nothing in either case.
Another thing that might cause confusion and mistakes is bleaching. When a snail lives in a very humid environment (and especially after the snail dies) the outer layer of the shell begins to degrade and turn white. Because of this you can find some nemoralis shells with seemingly white lips. In cases like that, try to locate the still intact parts of the shell and base the identification on them, if there are any. Bleaching can be recognized by how it makes the surface matte and chalky in texture.
These all have brown lips, but it's not so easy to see with all the bleaching. In cases like these, as always, it's useful to look closely. When you notice the bleaching on the outer shell, you should adjust your expectations of how dark the lip is going to be - very likely it too, will seem considerably lightened.

Do you think this is hortensis?

It is not.
Secondary Traits
So far we went over the primary identification characteristics - the love arrow cross-section and the lip color. What if a cepaea is juvenile? There's no lip so you can't use that, and there aren't going to be love arrows either and maybe it's a photo, so you couldn't see the love arrows anyway. DNA analysis is always possible, but far too fancy. Is it unidentifiable to the species then?
I think not. Hortensis and nemoralis differ not only in their primary traits, but in many other ways as well, and you can also use these differences for identification. Another reason why being aware of these secondary traits can be useful, is that they can help you spot the potential reverse color cepaeas, the ones where the color of the lip is atypical. If the secondary traits tell you one thing, and the lip color - another, tread carefully.
The more secondary traits you're aware of, the more effectively you can form a gestalt impression, and the more reliable it will be. However, identifying that way should be done cautiously - secondary traits aren't perfectly reliable and aren't always applicable. If they were, you would call them primary traits. Often the presence of a trait can tell you something, while its absence tells you essentially nothing either way.
*1. Cepaea hortensis rarely exhibits any banding pattern other than 12345 or 00000, therefore other patterns are fairly strong evidence of nemoralis. It can happen though - see the wikipedia gallery: https://commons.wikimedia.org/wiki/Cepaea_hortensis
On the other hand, the merging of bands seems more frequent in hortensis, especially the total merge, (12345).
This is what nemoralis banding patterns typically look like:

12045 - this is not very common, but it illustrates the variety of unusual banding patterns in nemoralis. [observation] [ImgLic]
Hortensis, on the other hand:

And a 12345. This (give or take band merging), and 00000 is what the vast majority of hortensis looks like. [observation] [ImgLic]

Sometimes bands become pale and reddish. In nemoralis this is possible, but rare. You should probably not describe this as 0.5, 1, 1.5, 2, 2.5. [observation] [ImgLic]
*2. In particular, the 00300 pattern is very common in nemoralis and especially rare in hortensis. It is so unusual, that when you see a cepaea with 00300 and a white lip I think it's pretty likely to be the reverse color nemoralis, actually. Meaning that the "00300 = nemoralis" correlation may just be more reliable than the "white lip = hortensis" correlation.
There are some photos of snails with white lips and 00300 pattern around:

This one is likely just a white lip nemoralis, misidentified. See the size, the location - over 2 cm, Pyrenees. [observation] [ImgLic]
Meanwhile on INat:
There's a number of observations of white-lipped 00300 cepaeas from the West of France, Paris and the Pyrenees that are identified as hortensis but are probably nemoralis in reality.
*3. A combination of the red base tone and the dark bands simply doesn't seem to occur in hortensis at all. It is therefore also very strong nemoralis evidence.
Hortensis can be red, but if it is, there will be no bands. I'm not sure if there exists any single photo of a white lipped cepaea with black bands on the internet, at all. If it does, I'm fairly sure it's reverse color nemoralis.
*4. Band number 5 being tighter in nemoralis, wider in hortensis - a reliable trait (or not?)
See this post for a discussion: apparently, not everyone agrees with this notion.
There's an idea that hortensis differs from nemoralis by the radius of the 5th band, sort of like this:
Here, I cherrypicked the specimens to make the difference easily visible. How reliable is this trait? Apparently, roughly this reliable:

These are the distributions of the band widths in the dataset that got. You can see that this trait is neither totally reliable nor totally useless.
*5. Nemoralis is bigger. This is a refreshingly simple way to distinguish the two species: they pretty clearly separate by size.

There's not a whole lot of overlap, meaning that almost all nemoralis are bigger than almost all hortensis. Still, there are always exceptional individuals and you often can't estimate the size very well when dealing with photos.
*6. Nemoralis is flatter, hortensis is closer to a ball shape. Ratio of height to width is usually higher in hortensis. That is another simple trait difference. Another subtle and not totally reliable trait, but sometimes it helps. Perhaps this is simply another manifestation of 1.5, the size difference. Maybe bigger cepaeas always tend to be flatter, and small ones - rounder.

On the left - reverse color nemoralis, on the right - hortensis. When the coloration is exactly the same, the eye can appreciate the difference in shape. From H. Zell image gallery.[ImgLic]
*7. Reddish or pink apex usually implies nemoralis. It is rarely seen in hortensis, except when the whole thing is red. Maybe this is just another way to state point 3.
Sometimes the apex of a cepaea shell has a color a little different from the general base tone - brighter and more saturated, like this:

There are exceptions, like this red-ish tipped hortensis, but this is uncommon. [observation] [ImgLic]
Reverse Color Cepaeas
Let's take a look at them, nemoralis first:

Nemoralis from Italy. White lip in nemoralis can go hand in hand with colorless bands. It's unclear whether this one was identified anatomically or not. Nemoralis like this one seems to be common in northern Italy, but very rare in most other places.[observation][ImgLic]
Here's a photo of a pair of white lipped nemoralis , these have been identified anatomically. Not much there that would have alerted the identifier except the size and the 5th band.
And now hortensis:

From Lviv. Image comes from this paper (the link seems not to work now) by Gural-Sverlova & Gural. Notice the 5th band.

I collected this one in cape Kolka, a location where brown lipped hortensis are known to occur, though I did not ID that one anatomically. The ID here is based on size and location among other things and is debatable. [observation]
More on INat: https://www.inaturalist.org/observations/161374476
You can see that none of these have a properly brown lip of solid dark color, such as nemoralis typically would have. Here, the color is lighter and you can see the white substance through it. Looking from the outside (from the side of the body whorl, that is) in many case the lip doesn't even look dark at all. This might give identifiers some hope that hortensis like that can be spotted with no dissection, perhaps.
How much of a problem are those, really?
Not that much of a problem, I think. At least, it's mitigated by two facts: 1) you have all the previously mentioned secondary traits that can help you out and alert you when the lip color is pointing the wrong way and 2) reverse color cepaeas seem to mostly be localized to specific areas. In some places they appear to be altogether absent.
Where I am, in Latvia that is, white-lipped nemoralis seems to not exist. I've never found any and there seems to be no specimens in the national museum. Latvia is by no means the only place like that. On INat all the observations of white lipped snails with the secondary nemoralis traits are concentrated in a few areas - Pyrenees, Northern Italy, west of France, and essentially nowhere else. There are almost none in North America. There must be more out there than I know about, going unnoticed, but they can't be common. Any population of white lipped nemoralis would be producing the suspicious white-lipped 00300 snails, and we see very few of those.
Brown lipped hortensis may be more troublesome. There are few good secondary traits indicating hortensis, and so the reverse color specimens are going to be more insidious and harder to sniff out. It's also unclear where they are localized, if anywhere. I don't think they can be common though, because if they were, we'd see lots of "transitional" hortensis with pale pink lips, somewhere between black and white, and we see very few. Likewise, there are probably very few brown lipped hortensis.
Afterword
It was possible to write this and assemble all these photos because people have been good enough to release them under the sufficiently permissive licenses. My life would have been even easier if all the photos were in public domain and then I'd save all those hours that needed to be spent on painstakingly providing attributions. The lesson here is: renounce copyright and make the whole world wealthier with every free photo. Your (c)-s will do you no good. They will, however, add an extra layer of difficulty to writing a (debatably) useful post.